Research
Designing Materials as Electrocatalysts
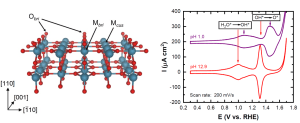
Transitioning to a carbon-zero economy demands innovative approaches for efficiently converting renewable energy into energy-storing compounds and essential building blocks for materials. Our group is committed to unraveling the characteristics that make an electrocatalyst effective for such vital transformations. To tackle this issue, we fabricate well-defined, single-crystalline films. These model electrocatalysts allow unparalleled control over surface terminations, strain, and chemical composition. Using these precisely engineered surfaces, we rigorously quantify aspects of surface thermodynamics such as electroadsorption [Ref], surface coverage [Ref], and corrosion behaviors [Ref]. This methodology has allowed us to critically test theories of heterogeneous catalysis and pin-point rate-limiting steps in complex multi-electron electrochemical reactions. Our strategy of employing thin films also enables us to tune the sub-surface chemistry, thereby gaining control over the surface’s electronic structure at the atomic level [Ref].
Tracking Dynamics on Surfaces
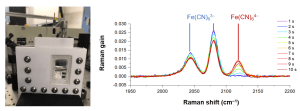
When electrons transfer at an electrochemical interface, a new bond may be formed while the old one dissociated. Our group is dedicated to probing the intricate bond formation and dissociation dynamics during electron transfer at electrochemical interfaces. We are particularly interested in the real-time mechanisms and dynamics behind electrocatalysis [Ref]. To achieve this, we employ femtosecond laser techniques such as Femtosecond Stimulated Raman Spectroscopy and Second Harmonic Generation. In the former, we have captured near-surface profiles of electron donors and acceptors during electrochemical reactions [Ref]. In the latter, we have explored the nature of the electric field at the solid-liquid interface to understand the electrical double layer [Ref]. Our goal to reveal how a surface becomes polarized during electrochemistry contributes to our understanding of bond formation and dissociation.
Extractive Materials Sciences
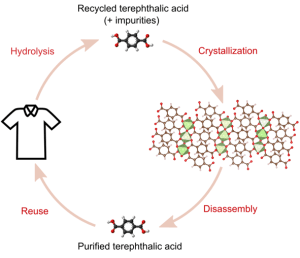
End-of-life products can be sources of valuable materials, from plastics to metals and semiconductors. Our research group is dedicated to advancing the knowledge of separation sciences to transform end-of-life products into resources. One key area of our recent work centers on applying reactive crystallization techniques to recycle textiles. In this approach, we extract polyester monomers from textile wastes, which often contain many impurities, such as dyes and additives. We assemble these monomers into metal-organic frameworks (MOFs) to purify them. This strategy crystallizes the monomers, rejecting contaminants from the depolymerization. The result is a highly effective extraction of high-quality polyester monomers from post-consumer textiles [Ref].
